Cis but 2 Ene
The branched isomers are 2-methylbut-1-ene 3-methylbut-1-ene isopentene and 2-methylbut-2-ene isoamylene. Position isomerism also called regioisomerism - constitutional isomers in which a functional group or substituent changes.
Out Of The Two Trans But 2 Ene And Cis But 1 Ene Which Is More Stable And Why Wired Faculty
Tissue-specific Gene Expression and Regulation TiGER.

. The proper name for this molecule is either trans-2-fluoro-3-methylpent-2-ene because the alkyl groups that form the backbone chain ie methyl and ethyl reside across the double bond from each other or Z-2-fluoro-3. You get a mixture of two isomers formed - cis-but-2-ene and trans-but-2-ene. Cis-2-Butene C4H8 CID 5287573 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
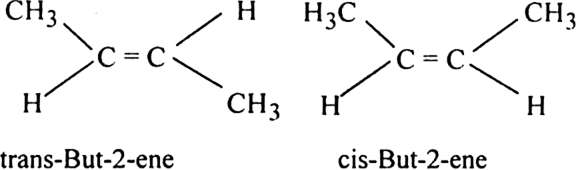
7023 7010 7035 2731 7249 9832 7544 7545 1585 SUBSTANCE DEA NUMBER CSA SCH NARC OTHER NAMES AB-FUBINACA N-1-amino-3-methyl-1-oxobutan-2-yl-. The products are but-1-ene CH 2 CHCH 2 CH 3 and but-2-ene CH 3 CHCHCH 3. Trans-but-2-ene is also known as E.
A simple example of cis-trans isomerism is the 12-disubstituted ethenes like the dichloroethene C 2 H 2 Cl 2. TiGER is a database developed by the Bioinformatics Lab at Wilmer Eye Institute of Johns Hopkins University. CH 3 2 CCHCHClCH 3 is 4-chloro-2-methyl-2-pentene.
The cis isomer of pent-2-ene has a boiling point of 37 o C but the boiling point of the trans isomer is 36 o The difference is small because the bond polarity is low. Skeletal isomerism also called chain isomerism - structural isomers in which components of the skeleton are arranged in a different order. Cis-2-Pentene is used in olefin metathesis.
In fact the situation is even more complicated than it looks because but-2-ene exhibits geometric isomerism. The Zaitsev Rule favors formation of 2-butene cis trans over 1-butene. This is most commonly seen when the skeleton or backbone consists of a carbon chain.
2-Pentene has two geometric isomers cis-2-pentene and trans-2-pentene. Due to the polar nature of the bonds in 12-dichloroethylene the boiling point of the cis isomer is 603 o C whereas that of the trans isomer is 475 o C the C-Cl dipole moments. Cis-but-2-ene is also known as Z-but-2-ene.
This is common for the carbon-carbon double and triple bonds which have the respective suffixes ene and yne. The database contains tissue-specific gene expression profiles or expressed sequence tag EST data cis-regulatory module CRM data and combinatorial gene regulation data. ヘキセンHexeneはC 6 H 12 という分子式を持つアルケンである hexという接頭辞は分子に6つの炭素原子があることを意味し-eneという接尾辞は2つの炭素原子が二重結合で結ばれるアルケンであることを意味している 鎖の中の二重結合の配置と幾何によりいくつかの異性体が存在.
Solution for H2Ni cis but-2-ene. The PEC diagram for the dissolution of the mineral chalcocite Cu2S in water is shown to the right. If you are.
As per EPC diagram the mixed state has higher potential energy that is increasing temperature wou. Halogens on the other hand do not have a suffix and are named as substituents for example.

Butene Formula Structure What Are The Isomers Of Butene Video Lesson Transcript Study Com

File Cis 2 Butene Svg Wikipedia

5 2 Geometric Isomers And E Z Naming System Chemistry Libretexts

Among Cis But 2 Ene Trans But 2 Ene Which One Is Polar Why Youtube
No comments for "Cis but 2 Ene"
Post a Comment